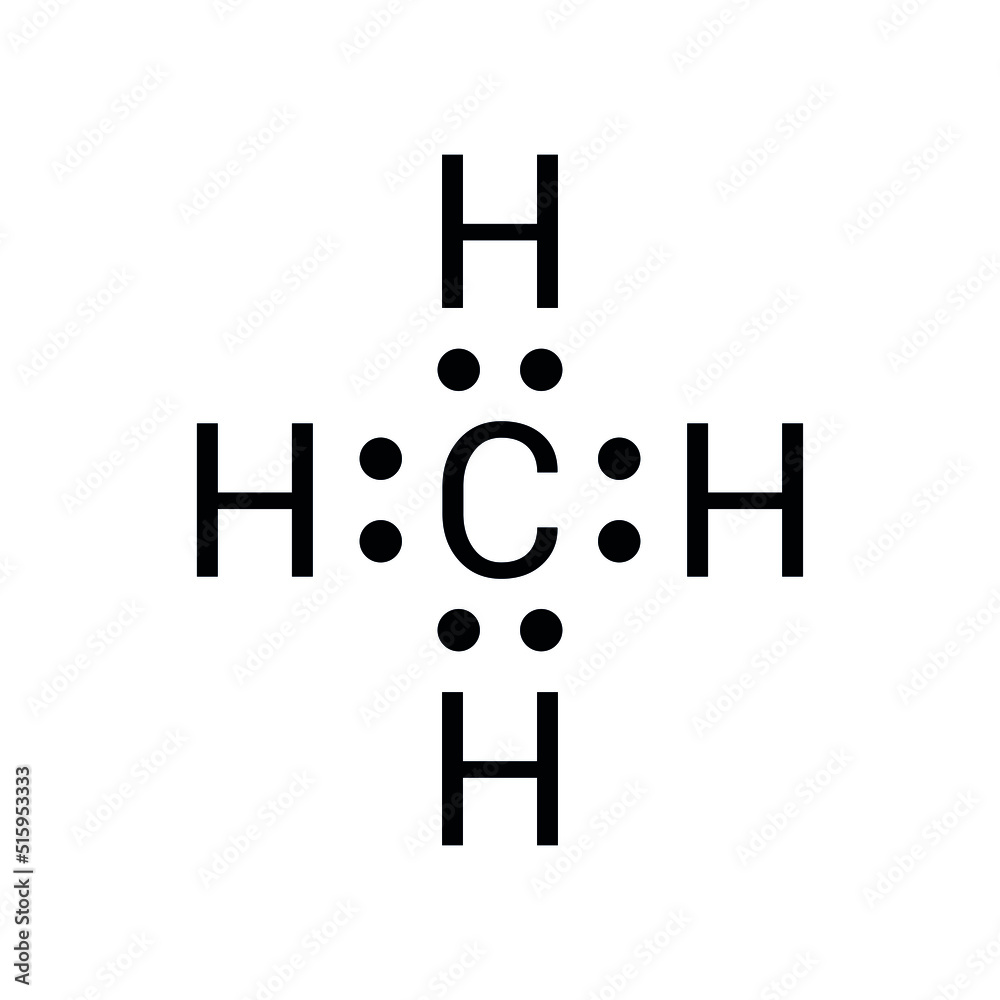
Ever wondered how the simplest molecule can unlock the secrets of the universe? Understanding the Methane Lewis Dot structure isn't just an academic exercise; it's the key to unraveling the complexities of molecular bonding and its impact on our world.
Methane, with its deceptively simple formula of CH₄, is a cornerstone of both organic chemistry and environmental science. The Lewis dot structure offers a clear visual representation of the bonds between the carbon atom and its four hydrogen partners, allowing us to grasp the molecule's behavior in diverse settings.
This exploration will dissect the Methane Lewis Dot structure, illuminating its practical applications and highlighting its importance in the broader field of chemistry. Whether you are a seasoned chemist or a curious student, this guide offers everything necessary to fully understand methane's electron distribution and molecular architecture.
- Breaking Jungkook Military Service Bts Future Amp Army Reacts
- Unlock Remote Iot Vpc Ssh Raspberry Pi Setup Free Guide
| Aspect | Details |
|---|---|
| Molecular Formula | CH₄ |
| Central Atom | Carbon (C) |
| Number of Valence Electrons | 8 (4 from Carbon, 1 from each Hydrogen) |
| Type of Bonds | 4 Single Covalent Bonds (C-H) |
| Molecular Geometry | Tetrahedral |
| Bond Angle | Approximately 109.5 degrees |
| Hybridization | sp3 |
| Common Uses | Fuel (Natural Gas), Production of chemicals, Energy source |
| Environmental Concern | Greenhouse Gas, Contributor to Global Warming |
| Reference Website | LibreTexts Chemistry |
- Andrew Tates Net Worth The Untold Story Of His Success
- Discover The Inspiring Journey Of Brandon Biggs Pastor A True Leader